A natural person may bring medicines into the country for personal use (for example, in traveller’s baggage) from other countries and receive medicines* by post. Please see detailed information in the infographic regarding bringing medicines from other countries and in the detailed overview below.
Infographic
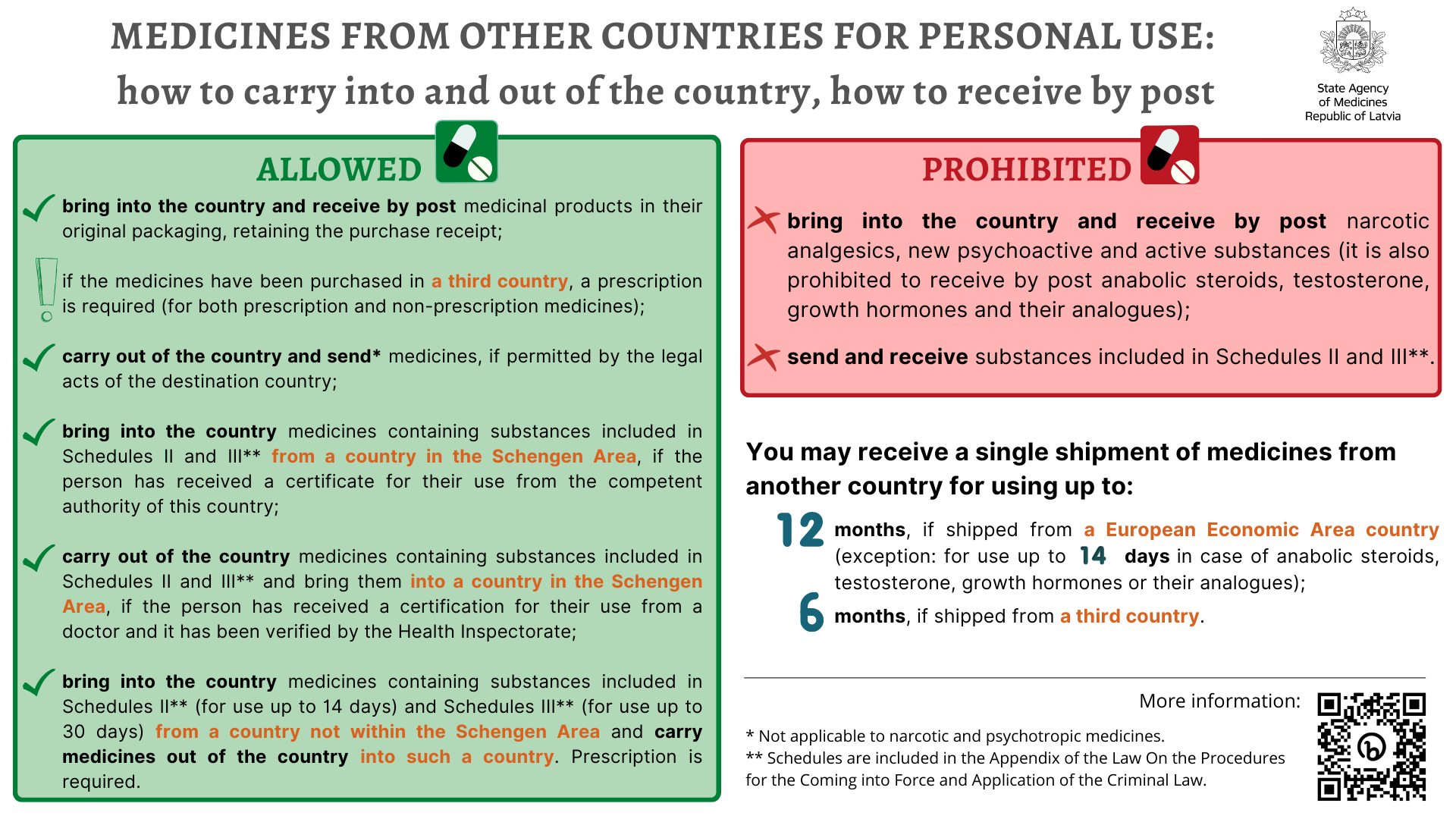
In addition, please note that medicines received by post from third countries are dispensed upon show of prescription or a document issued by a healthcare institution. For documents in a foreign language, a translation must be attached in compliance with the legal acts on procedure for verification of document translations in the official state language. This is applicable both to prescription and non-prescription medicines. Please note that you will be required to present a prescription issued by a doctor or a document issued by a healthcare institution at the post office of Latvia also in case you have ordered non-prescription medicines from another country.
A natural person is entitled to:
- Bring in from foreign states (for example, in a traveller’s baggage) or receive by post medicinal products for personal use*, if they are in the original packaging, their manufacturer and state of manufacture are identifiable on the packaging and they have a purchase cheque or an equivalent document;
- Export medicinal products for personal use or send medicinal products by post to another state, if it is permitted by the national law of the state to which medicinal products are delivered (checking any restrictions in the relevant country regarding import of medicines or sending medicines by post; not applicable to narcotic and psychotropic medicines);
- Enter from a country which is a Schengen Agreement country and bring into the country medicinal products for personal use which contain substances included in Schedules II and III if the person has received a certificate issued by the competent authority of the respective country for the use of narcotic or psychotropic substances for medical purposes.
- Upon leaving for a Schengen Agreement country, a citizen of Latvia, a non-citizen and a foreigner who has received a residence permit may carry medicinal products necessary for medical treatment which contain substances included in Schedules II and III if the respective person has received a certificate for the use of narcotic or psychotropic substances for medical purposes issued by a physician and approved by the Health Inspectorate in accordance with the laws and regulations regarding record-keeping of medical documentation.
- Enter from a country which is not a Schengen Agreement country or leave for such country and bring into or take out medicinal products containing substances included in Schedules II and III for personal use without a special permit if the medicinal products included in Schedule II are intended for a course of medical treatment not exceeding 14 days but the medicinal products included in Schedule III are intended for a course of medical treatment not exceeding 30 days. The need to use such medicinal products shall be certified by the person by presenting a prescription, a true copy or copy of the prescription or other documents that certify such fact.
A natural person is not allowed to:
- Bring in from another country narcotic analgesics, new psychoactive substances and active substances for personal use;
- Receive by post narcotic analgesics, new psychoactive substances and active substances, as well as anabolic steroids, testosterone, growth hormones or their analogues;
- Send and receive medicinal products included in Schedules II and III via inland or international postal services.
The amount of medicinal product for personal use in a single shipment from another country may be:
- For up to 12 months of use, if the medicinal product is imported or received by post from a European Economic Area country;
- For up to 14 days of use, if anabolic steroids, testosterone, growth hormone or their analogues are imported from a European Economic Area country;
- For up to 6 months of use, if medicinal product is imported from a third country.
If the amount of medicinal product brought in from another country or received by post exceeds three packaging units (primary, secondary) per each medicinal product, the person must certify the use of medicinal product by written certification indicating personal use of medicinal product and confirmation that the medicinal product is not a narcotic analgesic, new psychoactive substance, active substance, as well as an anabolic steroid, testosterone, growth hormone or their analogue (if received by post), and that the person takes full responsibility for use of this medicinal product. Import of anabolic steroids, testosterone, growth hormone or their analogue must be justified with a prescription or document issued by a healthcare institution.
Important!
In case of remote (online) purchase of medicinal products, the address of the pharmacy, licence number, pharmacy’s website address, institution issuing pharmacy licence and its address must be indicated on the postal package.
The abovementioned procedure for medicinal product circulation is stipulated by Cabinet regulation regarding the procedure for distribution and quality control of medicinal products and the law on legal trade of narcotic and psychotropic substances and medicinal products, as well as precursors.
*except narcotic medicinal products and psychotropic substances controlled in Latvia.
Additional information
Law On the Procedures for the Coming into Force and Application of the Criminal Law (in Latvian)
Law On the Legal Trade of Narcotic and Psychotropic Substances and Medicinal Products, and also Precursors (in Latvian)













