Foreign country packaging in Latvia
In accordance with legal acts regarding the procedure for labelling of medicinal products and requirements for package leaflets, the labelling and package leaflet of medicinal products distributed in Latvia must contain information in the state language (please, use official publication in Latvian for legal reference). In situations where the marketing authorisation holder (hereafter – MAH) is unable to ensure availability of medicinal products with labelling information and package leaflet in the state language (including repacking of medicinal product) exception can be made by distributing medicinal products in foreign packaging material.
Notification of distribution of medicinal products in foreign EU/EEA country packaging
On 1 January 2022 amendments to the Regulation No 57 of the Cabinet of Ministers of the Republic of Latvia "Regulations Regarding Procedures for the Labelling of Medicinal Products and the Requirements to Be Set for Package Leaflets of Medicinal Products", adopted on 17 January 2006 (hereafter - Regulation No 57), came into force, determining changes in distribution of medicinal products authorised in Latvia in packaging material intended for EU/EEA countries and cases where the MAH may notify the State Agency of Medicines (hereafter – the Agency) of such distribution without requesting the permit from the Agency or repackaging the product prior the distribution.
On 20 March 2024, the Regulations No 166 of the Cabinet of Ministers of the Republic of Latvia "Amendments to the Regulation No 57 of the Cabinet of Ministers of the Republic of Latvia "Regulations Regarding Procedures for the Labelling of Medicinal Products and the Requirements to Be Set for Package Leaflets of Medicinal Products", adopted on 17 January 2006””, adopted on 12 March 2024, came into force, expanding the exceptional cases when MAH no longer needs to request the permit from the Agency to distribute medicinal products in Latvia in foreign packaging.
From now on, MAH can also distribute medicinal products registered in Latvia in foreign packaging, by notifying the Agency - in cases, when no other medicinal products with identical active substance, dosage and medical form are included in the Medicinal Product Register of Latvia.
A flowchart in which cases a notification must be submitted and in which it is necessary to obtain the Agency's permit:
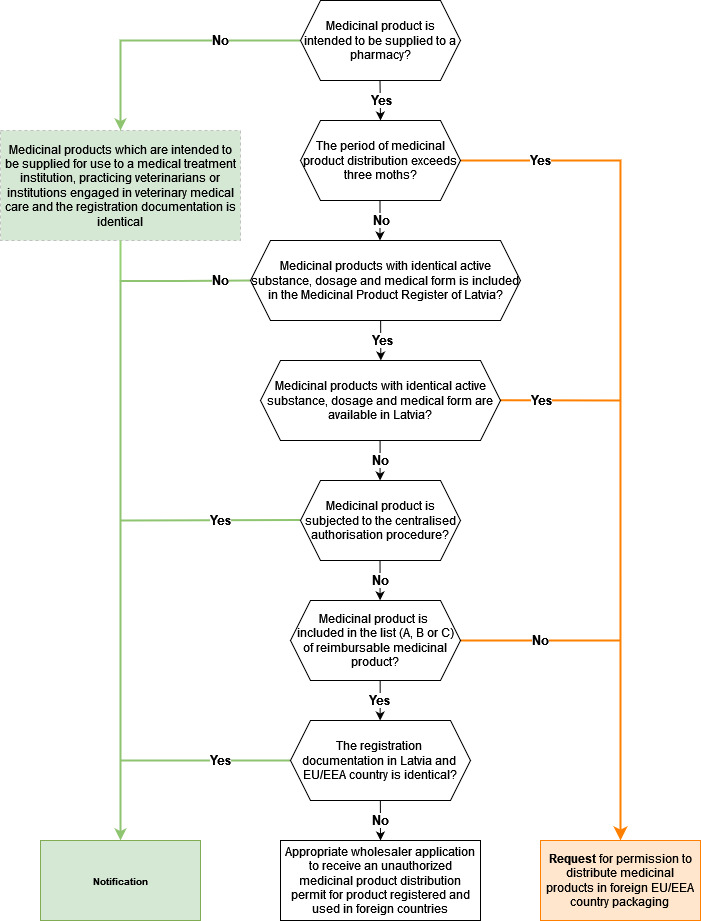
In the notification following information must be included:
- name of the medicinal product;
- strength, size of the package and amount;
- batch number and expiry date;
- country for which the medicinal product was intended for sale;
- any additional information (how long the product will be distributed in foreign packaging, when product with labelling information and package leaflet in the state language will be available, for example, by repackaging the product).
We would like to point out that that the MAH is responsible for ensuring wholesalers with needed amount of package leaflet and labelling translation in state language in each consignment so that:
- The supplier of the medicinal product shall ensure the necessary number of translations of the labelling and package leaflets in the state language according to the request of the medical treatment institution, social care institution, practising veterinarians or institutions engaged in veterinary medical care;
- When product is dispensed at pharmacy to patient, pharmacist, it’s assistant or health professional could add the labelling translation and package leaflet in state language, inform the patient that the product is in foreign packaging and explain how to use the product.
Please make sure that the translations of package leaflets and labelling text provided with the medicines are compliant with the requirements of normative acts regarding the procedure for labelling medicines and package leaflets of medicines (for example, making sure that these are not draft or project documents, the last date of approval of package leaflet is indicated, Agency’s contact information is indicated with respect to reporting adverse reactions, all of the information on the labelling applies to the specific product and does not include unnecessary information that may mislead the consumer), and that the general guidelines regarding the requirements for package leaflets and labelling text have been followed.
Please ensure that medicines wholesalers who are the recipients of the medicinal product packagings in question also receive a sufficient number of translations of the relevant package leaflet and labelling text that should be added to the packagings upon distribution.
Before sending the notification, please make sure that:
- The registration documentation in Latvia and EU/EEA country (in which packaging the product is intended to be distributed in) is identical;
- The period of products distribution in foreign packaging to pharmacies does not exceed in the Regulation 57 mentioned three months from the moment when the notification is received at the Agency and after this period availability of the product in official language will be restored (for example by repacking the product);
- medicinal products included in the Register that are also included in the list (A B or C) of reimbursable medicinal products with identical active substance, dosage and medical form are not available;
- in case if the notification to the Agency is submitted by local representative, valid power of attorney is applied to the Agency in advance or added to the notification.
To submit the notification to the Agency, please use the online form
Upon receiving the notification, the Agency checks the compliance of the provided information with the requirements of Regulation No 57. In cases when inconsistencies are detected, the MAH is informed. In cases when the received information corresponds to the notification, it is accepted and the MAH is entitled to start distributing of the medicinal product specified in the notification. The Agency publishes the information included in the accepted notification on its website within three working days after receiving the notification.
Receiving the permit to distribute medicinal products in foreign EU/EEA country packaging
In other cases, when the above-described criteria defined in Regulation No 57 are not fulfilled regarding notifications provided by the MAH to the Agency regarding the distribution of medicinal products in foreign packaging (for example, distribution to pharmacies in foreign EU/EEA country packaging longer than three months), distribution in foreign packaging is possible only after receiving the permit issued by the Agency.
The list of medicines, for whom the Agency has received a notification from the MAH or local representative or for whom the Agency has issued a permit for distribution of medicines in a packaging intended for the market of another country pursuant to Article 6.3 and 7.6 of Regulation No. 57, is available here.













