In accordance with the 13 April 2018 amendments to the Cabinet of Ministers Regulation No. 376 of 9 May 2006 “Procedure for Registration of Medicinal Products”* (subparagraph 122.1), changes have been made to the procedure how the marketing authorisation holder (MAH)/applicant shall be informed of approval or refusal of variations to marketing authorisation documentation.
Hereafter, all information regarding approved, refused or partially approved variations to marketing authorisation documentation will be published on the State Agency of Medicines’ (SAM) website. Please note, that this section includes information assembled starting from 13 April 2018.
By selecting various search criteria, clients can obtain information regarding the status of submitted variations.
a) The following search criteria may be used – medicinal product name, MAH name, authorisation number, procedure number, variation procedure number, “MAH transfer” designation, name of active substance.
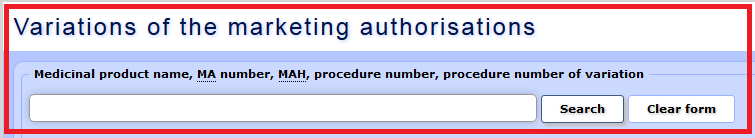
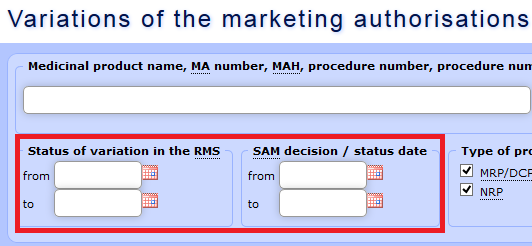
b) Data may be selected using the fields “Variation status RMS** date” and “SAM decision/status date”. For variation applications regarding medicinal products authorised in the mutual recognition procedure (MRP) or the decentralised procedure (DCP), the approval date indicated in the search form coincides with the date when the reference member state has informed the applicant regarding the fact (Subparagraphs 24.1.a, 24.3.a and 24.2.d of the Regulation 1234/2008).
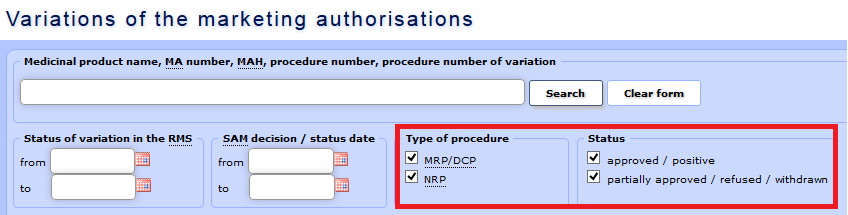
c) Data may be selected according to the type of procedure, i.e. MRP/DCP or NRP (national registration procedure), and according to status – approved, partially approved/refused/withdrawn. In case of partially approved variations, there will be two different statuses indicated in two separate rows for the relevant variation procedure:
 - approved/positive;
- approved/positive;  - partially approved/refused/withdrawn.
- partially approved/refused/withdrawn.
In case of refusal of variations to medicinal products authorised in the national procedure and MRP/DCP where Latvia is the reference member state, the marketing authorisation holder shall additionally receive the decision regarding refusal of variations.
* Cabinet of Ministers Regulation No. 210 of 10 April 2018 “Amendments to the Cabinet of Ministers Regulation No. 376 “Procedure for Registration of Medicinal Products””
** RMS – Reference Member State













