By selecting different search criteria, clients can obtain information regarding the date of marketing authorisation, renewal or withdrawal of marketing authorisation of medicines (medicines withdrawn from the Medicinal Product Register upon request from the marketing authorisation holder or due to expiration of marketing authorisation), or suspension of marketing authorisation of medicines.
a) You may use the following selection criteria – name of medicinal product, active substance, name/title of marketing authorisation holder, marketing authorisation number, procedure number.
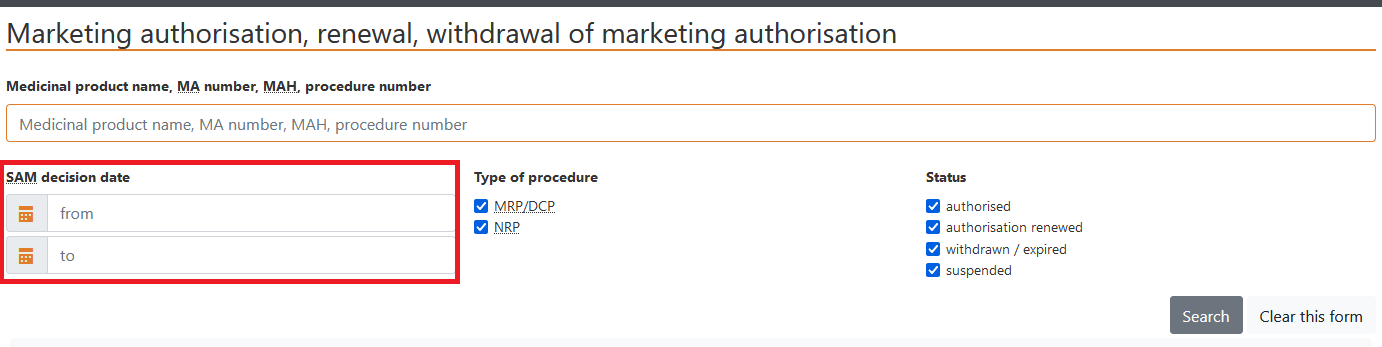
b) Data may be selected using the field “Date of SAM decision”.
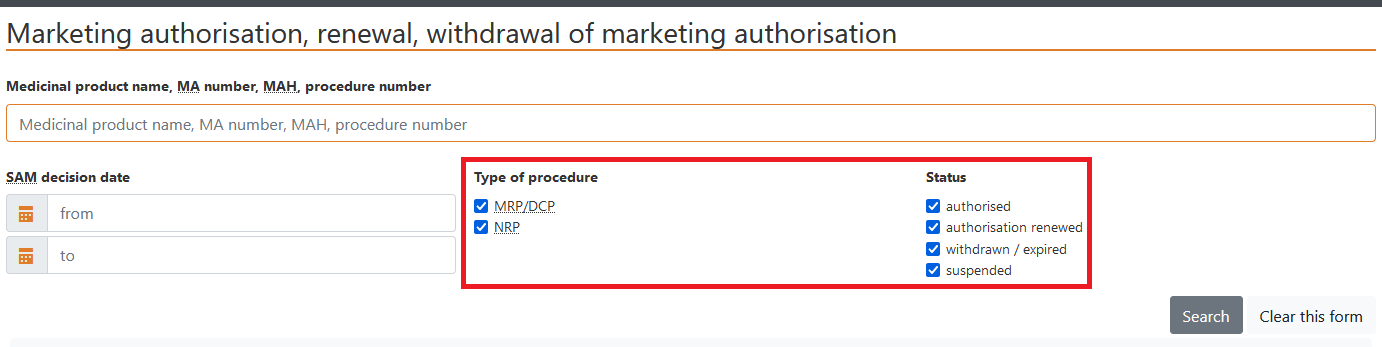
c) Data may be selected according to the type of procedure, that is, MRP/DCP or NRP (national procedure), or status – authorised, renewed, withdrawn/expired, suspended.
Status is depicted as follows: - authorised, renewed;
- withdrawn/expired,
- suspended.
Please note that information has been included on this page since 1 January 2019.
Information regarding authorisation, renewal and withdrawal of marketing authorisation of medicines in the Medicinal Product Register of Latvia until 1 January 2019, as well as information regarding the decisions adopted in relation to extension of marketing authorisation and not withdrawing from the Medicinal Product Register (Sunset clause) may be accessed here.













