Information on electronic reporting of adverse drug reactions
Electronic reporting of Individual Case Safety Reports (ICSRs)
The marketing authorisation holders should report ICSRs occurring in the territory of Latvia directly to the EudraVigilance Human Post Authorisation Module (EVPM) with the message receiver identifier EVHUMAN.
The marketing authorization holders shall electronically submit ICSRs to the Eudravigilance database:
- regarding serious suspected adverse drug reactions -within 15 days
- regarding non-serious suspected adverse drug reactions -within 90 days
Contact information for reporting ICSRs:
Kristine Plensnere, E-mail: kristine.plensnere@zva.gov.lv
Statistics of adverse drug reaction reports
The State Agency of Medicines of Latvia (SAM) has been maintaining the database for adverse drug reactions observed in Latvia since 2001, and since 2004 the reported information is being forwarded to the European Union database EudraVigilance for identification of new risks with medicines. Every year SAM analyses the information entered into the database in Latvia and the reporting activity in Latvia.
In 2017, the number of adverse drug reaction reports received in Latvia reached 588. In relation to the new reporting system introduced in in EudraVigilance 2017 and laid down in European normative acts, the number of reports submitted by marketing authorisation holders also increased.
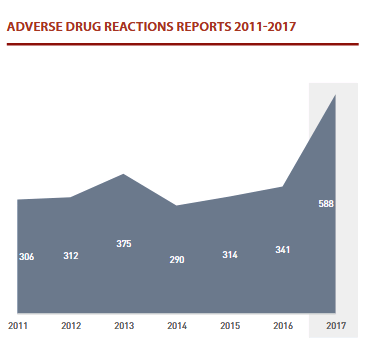
Doctors and pharmacists submitted 49 reports. The Centre for Disease Prevention and Control (CDPC) submitted 34 reports on adverse reactions to vaccines.
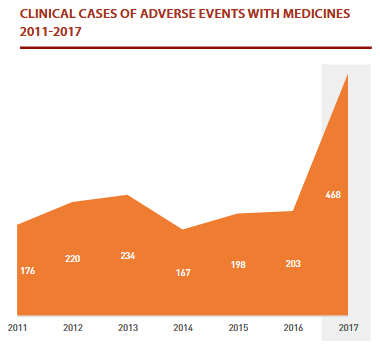
The number of clinical events reported to SAM in 2017 increased, reaching 468 events.